-
Property & Casualty
Property & Casualty Overview

Property & Casualty
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.
Expertise
Publication
Biometric Information Privacy – Statutes, Claims and Litigation [Update]
Publication
Inflation – What’s Next for the Insurance Industry and the Policyholders it Serves?
Publication
Human Activity Generates Carbon and Warms the Atmosphere. Is Human Ingenuity Part of the Solution?
Publication
Illinois Changes Stance on Construction Defect Claims – The Trend Continues
Publication
Generative Artificial Intelligence in Insurance – Four Aspects of the Current Debate
Publication
Battered Umbrella – A Market in Urgent Need of Fixing -
Life & Health
Life & Health Overview

Life & Health
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.

Publication
Underwriting High Net Worth Foreign Nationals – Considerations for U.S. Life Insurance Companies
Publication
Group Term Life Rate & Risk Management – Results of 2023 U.S. Survey
Publication
Trend Spotting on the Accelerated Underwriting Journey
Publication
All in a Day’s Work – The Impact of Non-Medical Factors in Disability Claims U.S. Industry Events
U.S. Industry Events
Publication
Marginal Gains in the Medicare Supplement Market -
Knowledge Center
Knowledge Center Overview

Knowledge Center
Our global experts share their insights on insurance industry topics.
Trending Topics -
About Us
About Us OverviewCorporate Information

Meet Gen Re
Gen Re delivers reinsurance solutions to the Life & Health and Property & Casualty insurance industries.
- Careers Careers
Preventing Dementia Through Mid-Life Interventions

January 07, 2019
Dr. Chris Ball
English
Français
There has been a significant change in thinking about the problems posed by dementia (chronic progressive neurodegeneration causing decline in cognitive function) by both researchers and the wider health promotion community over the last few years.
In the early 1990s although knowledge of dementia risk factors was emerging, it was concluded that there was no justification to conduct trials or introduce large-scale interventions to prevent the illness.1 Over the following 20 years, the focus was primarily on developing treatments for the pathological brain functions identified as the cause of cognitive decline. In part this was because the risk factors identified, old age, genetics and head injury, were not felt to be amenable to change, but it was also because the drive to develop pharmacological treatments, based on the cholinergic and amyloid hypotheses, was very strong.
The failure of these paradigms to deliver meaningful treatments and the recognition that the incidence of dementia has been declining in certain communities led to rethinking the approach, with a move away from treatment towards prevention.2 The discovery that biomarkers of disease show changes many years prior to the development of clinically significant symptoms suggested that interventions must be made much earlier if the disease is to be treated or, more importantly, prevented.
The scale of the problems associated with an aging population, the development of dementia and provision of care are well known; currently 850,000 people in the UK and 47.5 million worldwide live with dementia. These figures could rise to 2 million and 150 million respectively by 2050.3 The numbers translate not only into significant direct and indirect care costs (higher than the costs of cancer, heart disease or stroke) but also very considerable individual suffering. In the absence of effective treatments even relatively small reductions in the incidence of the disorder or delaying the onset of clinical symptoms would reap significant benefits.
It has been difficult to develop a “return on investment (ROI)” tool to quantify the financial effectiveness of interventions as few studies are analysed in this way. The evidence can be difficult to interpret because studies often include both younger and older people, use cognitive change rather than developing dementia as their outcome measure, or have not been in place for long enough to understand their full implications.4 Despite these caveats it has been argued that by implementing “best-practice” interventions to reduce risk factors the UK could save £42.9 billion by 2040 (minus the cost of the interventions).5 However the data is interpreted there is a broad consensus that about one third of dementia cases might be attributable to “potentially modifiable” risk factors. A 20% reduction in these risk factors per decade would reduce the UK prevalence by 300,000 (16.2%) cases by 2050.6 There have been a number of reviews published in recent years, each offering a differently nuanced take on the issue. Whilst more reviews should not be equated with more evidence, it is an indication that prevention is seen as increasingly important and achievable. More recently the UK government has recommended that advice about dementia prevention should be included in the routine over 40s health check.7
Taking a lifestage approach means considering interventions when it is unlikely that many people would be thinking about developing dementia. Providing education beyond primary school level is probably the most powerful intervention globally. Fewer years of education is associated with a Relative Risk (RR) of developing dementia of 1.59 (95% CI 1.26–2.01). Because worldwide the estimated prevalence of poor education is so high (40%) this leads to a high Population Attributable Fraction (PAF) for dementia.8 However, this discussion will concentrate on mid-life interventions that have the potential to be promoted through insurance channels.
Cardiovascular Health
Several reviews have identified a series of risk factors focusing upon the cardiovascular health (see Table 1).9
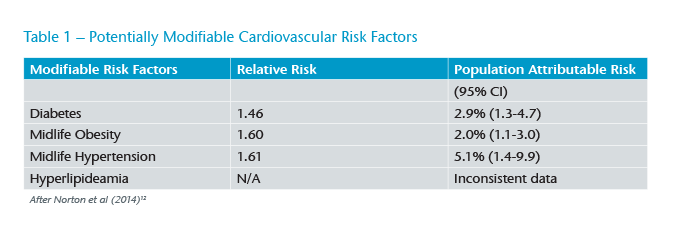
The widespread introduction of programmes to reduce heart attack and stroke by ensuing good cardiovascular health in mid-life (age 40-64) are probably responsible for the reductions seen in the incidence of dementia (“What’s good for the heart is good for brain”) in some markets.10 Changes in vascular risk have been implicated not only in Vascular Dementia (VaD) but also Alzheimer’s Disease (AD). In clinical practice many patients who undergo scanning have evidence of changes consistent with both of these common forms and are classified as having Mixed Dementia (see Figure 1).
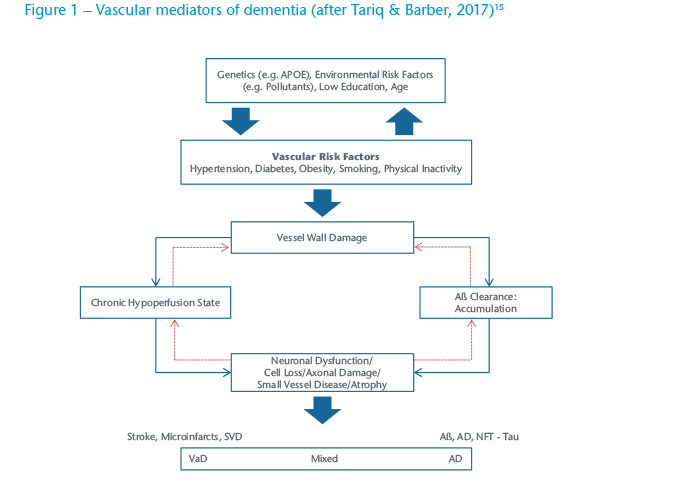
Although each of these factors has a relatively small PAF (possibly in part due to the relative success of these programmes) the potential combined impact remains large and generates added impetus to the programmes.11 The impact of the growing numbers of obese people will have on dementia incidence remains to be seen as, in older age, obesity appears to have a mildly protective effects against the development of dementia.14
The second group identified for potential interventions are intimately involved with “lifestyle” where there may be more scope for intervention (see Table 2).
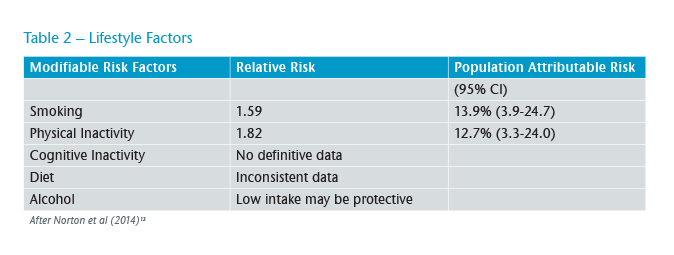
Physical inactivity is highly prevalent in many societies. Only 39.2% of German adults reach recommended levels of physical activity. More concerning in this context is that only 27.5% of adolescents attained the levels.16 There is evidence that mid-life inactivity increases the risk of developing dementia and that engaging with physical activity during mid-life is protective against developing the disease. This makes it an attractive target for intervention although the evidence suggests it is difficult to get over 50s to improve their physical activity in a sustained manner.17
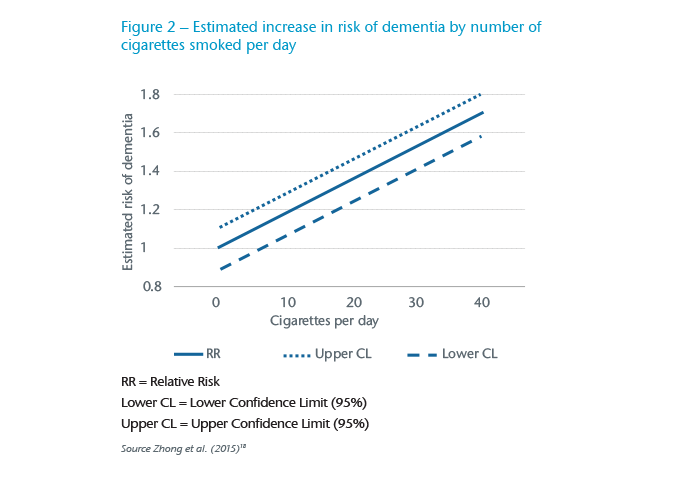
Some early work argued that smoking might actually be protective against the development of AD but the situation has subsequently become much clearer. Current smoking increases the risk of all causes of dementia whilst smoking in the past does not increase the risk. With 20% of the UK population continuing to smoke, the PAF is high meaning significant reductions in dementia could be achieved. What is not yet clear is when and for how long people need to be cigarette-free to reduce their dementia risk in old age (see Figure 2).
There is some debate about the importance of alcohol as a risk factor for dementia.19 Handing et al. (2015) calculated a J-shaped curve for the risk of dementia in relation to mid-life consumption over a 43 year follow-up (see Figure 3)20, a finding broadly supported in meta-analytical studies.21 This has meant that alcohol was not considered as a significant risk factor in some analyses and the advice to reduce intake was rather muted.22
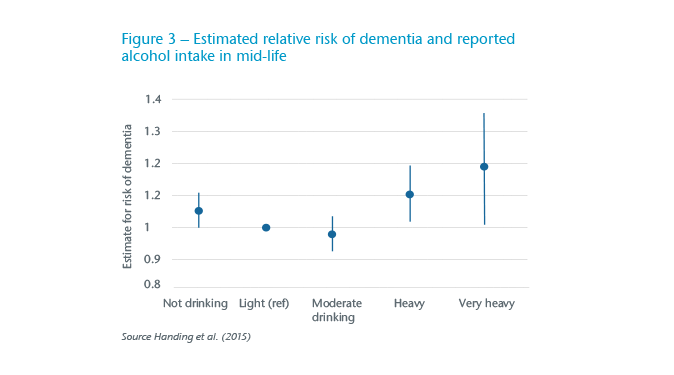
This approach does not do justice to the harms identified by Schwarzinger et al. (2018) who reported that 57% of those developing early-onset dementia also had alcohol use disorders.23 It is likely this group had co-morbidities that might also predispose them to developing dementia.24 Arguing that your next glass of wine is going to help prevent dementia will not be very persuasive.
The effects of diet in mid-life on the development of dementia are much debated. Read et al. (2017) concluded that the evidence is sparse because the majority of studies concentrate on immediate health benefits rather than long-term outcomes.25 However the “promoting brain health” team concluded that regular vegetable consumption and a Mediterranean diet may protect against dementia, particularly AD. The evidence for the benefits of vegetables is more convincing than it is for fruit.26 A better diet probably works by developing cardiovascular health and a healthy weight. The size of the impact of changing diet is not clear although it clearly has a relationship with obesity and diabetes.
There is an association between mid-life mental activities and lower risk of dementia in later life much of which is explained by early education and developing a cognitive reserve. Even for those with less education working with more complex data has the potential to reduce the risk. Although the studies are inconsistent in their methodology and outcomes there is enough evidence to give some support to the “use it or lose it” rubric.
Other potential interventions
The association between dementia and depression has been well described and is probably strongest for those who experience late-life depression.27 For some, it is a clear prodrome to the onset of cognitive impairment. For those who experience mid-life depression alone, the risk of developing dementia increased by about 20%.28 It is not known if active treatment would have any impact on the risk. More broadly described mental distress has been identified as a potential risk factor but the evidence is relatively weak, particularly as there is likely to be an overlap with depression, and the impact of programmes to reduce stress have not been reported.29
As with other “well-being” measures the associations between social isolation and loneliness and dementia are more robust in old age than in mid-life. Common sense suggests that a life-long pattern of active engagement outside the home would be likely to persist into older adulthood where the impact might be felt but the evidence that problems in mid-life are predictive of developing dementia is not robust.30
The concern about repeated head injuries in collision sports is a reflection of growing awareness of the cumulative damage repeated concussions may cause. Looking at broadly defined head injury the pooled relative risk (RR) estimates showed that head injury significantly increased the risks of any dementia (RR = 1.63, 95% CI 1.34–1.99).31 The long term impact of implementing standardised concussion assessment protocols such as the SCAT3 is not known but can only be positive.32
Issues such as quality of sleep and air experienced in mid-life may have an impact on developing dementia in late-life but like many of the other potentially modifiable risk factors evidence is not good but the arguments for generally improving health through interventions is strong for both short and long term benefits.
Conclusions
Dementia, in all its myriad forms, has no treatment that is effective once clinical evidence of the syndrome has emerged. This does not mean that significant steps cannot be taken to improve the quality of life of those living with the illness and their carers. Medical interventions at the pre-clinical phase may be on the horizon but interventions made in early and mid-life can happen today and significantly reduced the risk of developing dementia.
The insurance industry has engaged heavily in changing its relationship with their customers in recent years, developing relationships through rewarding healthy behaviours and providing well-being interventions well beyond their traditional remit. To date the importance of interventions to preventing dementia have not been stressed, partly perhaps because the people who might benefit from these interventions are not necessarily considering the consequences of their behaviours into very old age. However, as more people become concerned about this issue, and products develop to reflect the risks of longevity (long-term care riders to whole of life policies for example), the opportunity for insurers to play a role in dementia prevention becomes a reality. Although many companies are providing well-being services to their workers, the fluidity of the employment market allows insurance firms to provide continuity in this area.
In order to prevent dementia it is likely that a multipronged approach will be required and no single intervention will be adequate. The engagement of insurers with their clients as they develop their families and careers offers a unique opportunity to intervene positively to reduce the suffering from dementia in years to come.
Download PDF Version for Endnotes.





