-
Property & Casualty
Property & Casualty Overview

Property & Casualty
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.
Expertise
Publication
Structured Settlements – What They Are and Why They Matter
Publication
PFAS Awareness and Concern Continues to Grow. Will the Litigation it Generates Do Likewise?
Publication
“Weather” or Not to Use a Forensic Meteorologist in the Claims Process – It’s Not as Expensive as You Think
Publication
Phthalates – Why Now and Should We Be Worried?
Publication
The Hidden Costs of Convenience – The Impact of Food Delivery Apps on Auto Accidents
Publication
That’s a Robotaxi in Your Rear-View Mirror – What Does This Mean for Insurers? -
Life & Health
Life & Health Overview

Life & Health
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.

Publication
Key Takeaways From Our U.S. Claims Fraud Survey
Publication
Favorite Findings – Behavioral Economics and Insurance
Publication
Individual Life Accelerated Underwriting – Highlights of 2024 U.S. Survey
Publication
Can a Low-Price Strategy be Successful in Today’s Competitive Medicare Supplement Market? U.S. Industry Events
U.S. Industry Events
Publication
The Latest in Obstructive Sleep Apnea -
Knowledge Center
Knowledge Center Overview

Knowledge Center
Our global experts share their insights on insurance industry topics.
Trending Topics -
About Us
About Us OverviewCorporate Information

Meet Gen Re
Gen Re delivers reinsurance solutions to the Life & Health and Property & Casualty insurance industries.
- Careers Careers
Understanding Viral Hepatitis - What’s Relevant to Underwriting?

January 22, 2020
Dr. Sandra Mitic,
Annika Schilling
English
Français
Prevalence, current therapies and prevention are the key themes for insurers that are reviewing their approach to applications with a history of viral hepatitis.
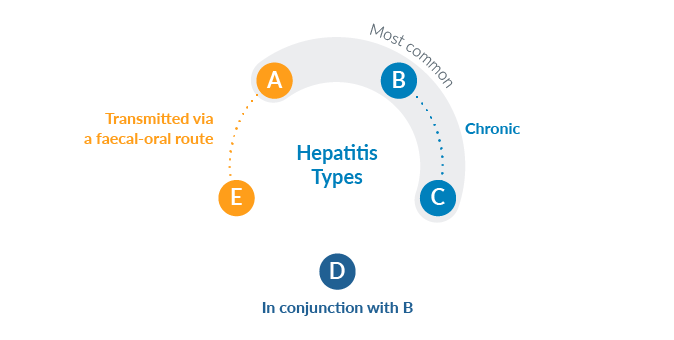
As its name suggests, viral hepatitis is the term used to describe diffuse inflammation of the liver triggered by a viral infection. The disease has five types - from A to E - each defined by a particular pathogen. The most common forms are hepatitis A, B and C.
Hepatitis A and E, which are transmitted via a faecal-oral route initiated by hand contact, nearly always resolve without consequences and are therefore not relevant from the Insurance Medicine perspective.
Types B and C are especially relevant, however, because of their possible chronic course. Hepatitis D occurs only in conjunction with hepatitis B infection and leads to particularly serious progressions. These three forms, individually and together, are transmitted through contact with blood or other body fluids.
Hepatitis can be asymptomatic and go unnoticed. It may also start with completely non-specific flu-like symptoms, gastrointestinal complaints and joint pain. The typical signs of liver disease - such as jaundice, enlargement of the liver and/or of the spleen and swelling of the lymph nodes - can develop subsequently.
The inflammation can cause liver cells to rupture and, as the condition increases in severity, bile can accumulate (cholestasis), leading to an increase in the level of bilirubin. Where an acute infection becomes chronic, the risk of scarring of the liver (cirrhosis) as a result of recurrent inflammatory processes. Finally, reduced synthesis output of the liver can occur.
Hepatitis B is one of the most common infectious diseases worldwide. Around two billion people have already experienced or are currently experiencing infection. Some 240 million people (around 3%) are chronically infected with the hepatitis B virus worldwide1 and 15 million in Europe2.
The hepatitis B virus cannot be completely eliminated with currently available therapy. This is because, as yet, no active substances are available that can prevent the virus from entering the liver cells and remaining there. Consequently, even after an infection has resolved and sufficient antibodies against the surface antigen have formed, replicable DNA copies of the virus remain in the liver cells in the form of mini-chromosomes.
Around 71 million people worldwide3 and 14 million in Europe are affected by hepatitis C4. In the U.S., 2.4 million people are living with it.5 Globally, about 1.75 million people are infected each year, which corresponds to a falling infection rate overall.6 Vaccination is not currently available.
Hepatitis C is relevant in the context of Insurance Medicine mainly because of its high chronification rate and the high risk of liver cirrhosis. After 20 years of chronic infection, the incidence of cirrhosis is 20%, and after 30 years it is over 40%. The annual rate for the development of HCC is 2%-4% when cirrhosis is also present.
WHO programmes to reduce viral hepatitis
Worldwide, hepatitis prevention programmes have been implemented to respond to epidemic hepatitis. The WHO developed one of the first global strategies on viral hepatitis, aiming to eliminate the disease by 2030.
The programme’s objectives include reducing new cases of chronic viral hepatitis B and C infections and minimising mortality due to infections. The key measures are:
- Comprehensive hepatitis B childhood vaccine coverage
- Prevention of hepatitis B virus mother-to-child transmission by birth-dose vaccination coverage
- Earlier viral hepatitis B and C diagnosis
- Broad access to viral hepatitis B and C treatment
- Measures for safe injections
- Measures for blood safety
The expansion of vaccination programmes and other measures have successfully reduced the number of endemic areas in the world.
Chronic hepatitis and underwriting
Although hepatitis B infection cannot be cured with current therapies, virological or immunological control of viral replication is almost always possible.
In the case of chronic hepatitis C, the use of combination drug therapy can completely eliminate the virus from liver cells - for certain genotypes. Moreover, with virostatic therapy using DAAs (direct-acting antivirals) a high possibility exists to even reverse fibrotic changes to the liver. The new DAA-based medication has revolutionised the therapy of hepatitis C patients. For a long time, only a fraction of patients with hepatitis C could be cured, whereas the chances of cure have now risen to over 90%, thanks to new drugs. In the next few years it may even be possible to cure almost every infected person.
For applications indicating a history of hepatitis B, C or D infection, the underwriter’s target is to identify inflammatory activity, unfavorable risk factors and potential long-term effects. Abdominal ultrasound and the Metavir-Score can give information about underlying changes of liver function in people with a history of viral hepatitis.
The applicant’s age, viral co-infections, alcohol consumption and ALT levels can be used as supportive evidence for risk assessment. Increased levels of hepatitis B DNA or hepatitis C RNA can be interpreted as a sign of active virus replication.
Gen Re recently updated our international underwriting guidelines for hepatitis, which are detailed in our CLUE underwriting manual. The main focus of the revision was to analyze new findings from studies in clinical medicine and see if new insights into the course of the disease require a change in underwriting assessment. The revision resulted in guidelines with partially adapted assessment criteria but no significant differences in the final ratings. Although the number of new hepatitis cases, of chronic hepatitis in particular, has decreased, the prognosis for all forms of the disease has remained largely stable over the past few years.
What is changing and increasingly diverse, however, is the information available at underwriting stage. Hepatitis therefore serves as a good example for one the basic principles of underwriting: The more information that is available about the risk to be insured, the more precise the individual risk assessment becomes. Very favorable assessments are possible even in the case of chronic disease, if the necessary clarity on the course and current status of the disease has been achieved.
But even if this information is not always available (and it is not always possible to obtain a complete picture without complicating the application process), the situation can be countered by a flexible design of underwriting guidelines, so that at the end of each underwriting case there is an assessment that does justice to the interests of both parties involved.
Endnotes
- Mitic’s claim that 240 people worldwide have Hepatitis B came from this link: https://www.medscape.com/answers/775507-38259/what-is-the-prevalence-of-viralhepatitis-globally (accessed 9 October 2019).
However, Medscape also says the prevalence for HVB worldwide is 350M at this link: https://www.medscape.com/answers/775507-38261/what-is-the-prevalence-of-the-hepatitis-b-virus-hbv-infection.
WHO said 257M had hepatitis B worldwide in 2015 https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
CDC says current estimate is 350M worldwide https://www.cdc.gov/hepatitis/hbv/pdfs/hepbatrisk.pdf. - Hepatitis B in Europe: http://www.euro.who.int/__data/assets/pdf_file/0007/377251/Fact-Sheet-Hepatitis-B_2019-ENG.pdf?ua=13. Hepatitis C worldwide: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c; https://emedicine.medscape.com/article/775507-overview#a5.
- For Hepatitis C in Europe, the WHO contradicts itself by a discrepancy of 1M, stating 15M in Europe as of July 2019 on its website (www.euro.who.int/en/health-topics/communicable-diseases/hepatitis/data-and-statistics); and 14M in its 2019 factsheet (www.euro.who.int/__data/assets/pdf_file/0009/377253/Fact-Sheet-Hepatitis-C_2019_ENG.PDF).
- https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm
- WHO estimates that in 2015, there were 1.75 million new HCV infections in the world https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.






