-
Property & Casualty
Property & Casualty Overview

Property & Casualty
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.
Trending Topics
Publication
That’s a Robotaxi in Your Rear-View Mirror – What Does This Mean for Insurers?
Publication
Cat Bonds – A Threat to Traditional Reinsurance?
Publication
Decision-Making in the Age of Generative Artificial Intelligence
Publication
Buildings Made of Wood – A Challenge For Insurers?
Publication
The CrowdStrike Incident – A Wake-Up Call for Insurers?
Publication
PFAS Awareness and Concern Continues to Grow. Will the Litigation it Generates Do Likewise? -
Life & Health
Life & Health Overview

Life & Health
We offer a full range of reinsurance products and the expertise of our talented reinsurance team.
Training & Education
Publication
When Actuaries Meet Claims Managers – Data-Driven Disability Claims Review
Publication
Chronic Pain and the Role of Insurers – A Multifactorial Perspective on Causes, Therapies and Prognosis
Publication
Fasting – A Tradition Across Civilizations
Publication
Alzheimer’s Disease Overview – Detection and New Treatments
Publication
Simplicity, Interpretability, and Effective Variable Selection with LASSO Regression Moving The Dial On Mental Health
Moving The Dial On Mental Health -
Knowledge Center
Knowledge Center Overview

Knowledge Center
Our global experts share their insights on insurance industry topics.
Trending Topics -
About Us
About Us OverviewCorporate Information

Meet Gen Re
Gen Re delivers reinsurance solutions to the Life & Health and Property & Casualty insurance industries.
- Careers Careers
The Latest in Obstructive Sleep Apnea
December 05, 2024
Dr. Jonah Fox
English
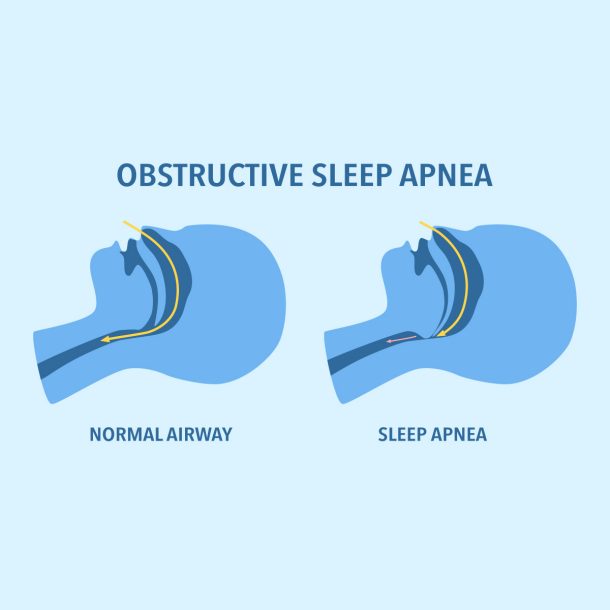
Obstructive sleep apnea (OSA) is defined as recurrent episodes of airway obstruction during sleep, resulting in reduced airflow despite ongoing respiratory efforts, leading to oxygen desaturation and waking. Approximately one billion people are estimated to have OSA across the globe,1 making it the most common respiratory-related sleep disorder.
While older adults are more likely to have obstructive sleep apnea than their younger counterparts, OSA is thought to be considerably underdiagnosed in the senior population.
Research suggests that 56% of people ages 65 and older have a high risk of OSA, but that only 8% of those have been tested for OSA.2 Obstructive sleep apnea is directly linked to daytime somnolence, cognitive impairment, and decreased quality of life, and has also been associated with broader health conditions, including high blood pressure, heart attacks, heart failure, and diabetes.
Patho-anatomy
Obstructive sleep apnea may be due to a number of factors, including decreased ventilatory drive, neuromuscular factors, and anatomic features. Certain anatomic risk factors, such as large neck circumference, bone, and soft tissue, can lead to increased upper airway pressure and insufficient space to accommodate airflow during sleep.
Upper airway muscle tone plays a significant role, and when these muscles are weakened, airway collapse can occur.
Anatomic Risk Factors
- Micrognathia, retrognathia
- Facial elongation
- Mandibular hypoplasia
- Adenoid and tonsillar hypertrophy
- Inferior displacement of the hyoid
Risk Factors
A number of risk factors for OSA have been identified, and include, but are not limited to, those listed below.
|
Increased BMI (>30) |
Friedman Tongue Position of FTP III or higher |
Other structural abnormalities that constrict the upper airway |
Hereditary factors |
|
Enlarged neck perimeter (>17 in for males, >16 in for females) |
Marked retrognathia |
Smoking |
|
|
Mallampati Score of III or higher |
Crowding of the oropharynx |
Alcohol |
|
Diagnosing Obstructive Sleep Apnea
A diagnosis of obstructive sleep apnea is most frequently made through a combination of subjective assessments (questionnaires, symptoms, etc.) and a physical exam, with an overnight polysomnography (PSG) being the gold standard for a proper diagnosis. A home sleep apnea test (HSAT) is another tool that is frequently used to diagnose OSA; however, unlike the PSG, it does not include an electroencephalogram. This makes it challenging to know if the patient was sleeping or not, thus frequently requiring the provider to rely on asking the patient about the subjective nature of their sleep following the study.
Examples of OSA risk assessment questionnaires include the Epworth Sleepiness Scale and the STOP‑Bang questionnaire.
The Epworth Sleepiness Scale consists of a series of questions related to sleepiness onset.
Responses are graded from 0 to 3, with 3 representing increased risk of OSA. Total scores of 10 or higher are suggestive of OSA.
The STOP-Bang questionnaire is a set of yes/no questions based on the STOP-Bang acronym:
S – snoring
T – tiredness
O – observed pauses in breathing
P – high blood pressure
B – BMI > 35 kg/m²
A – age > 50 years
N – neck circumference
G – male gender.
Five or more “yes” responses are considered high risk for OSA.
An overnight polysomnography is performed in a healthcare facility and utilizes a number of components (e.g., electrocardiogram, electroencephalogram, electrooculogram, electromyography, respiratory rate, tidal volume, and inspiratory and expiratory volumes) in order to calculate an apnea-hypopnea index (AHI). The AHI indicates the number of apneas (when breathing stops during sleep) and hypopneas (reduced breathing with decreased oxygen) per hour of sleep. An AHI ≥5 and <15 represents mild OSA, ≥15 and <30 represents moderate OSA, and >30 is representative of severe OSA.
While an overnight PSG is the gold standard for diagnosing OSA, home sleep apnea tests are also an option for diagnosing OSA; they are performed unattended in the patient’s home with a portable monitoring device.
Complications
Obstructive sleep apnea has the potential to impact cardiovascular health, mental health, and overall quality of life. Decreases in blood oxygen levels, which are common in obstructive sleep apnea, can increase blood pressure. High blood pressure, or hypertension, can in turn increase the risk of heart disease.
Perhaps unsurprisingly, OSA has been linked to an increased risk of myocardial infarction (heart attack), coronary artery disease, cerebrovascular accident (stroke), and heart failure. From a behavioral health perspective, studies have found that adults with sleep apnea “have a relatively high prevalence of depressive symptoms, and that the severity of sleep apnea positively corelated with the depressive symptoms.”3
While somewhat obvious, sleep apnea also increases daytime sleepiness, irritability, and fatigue. This may lead to falling asleep during the day, difficulty concentrating, and an increased risk for automobile accidents. Obstructive sleep apnea, and related snoring, may also impact the sleep and quality of life of those around the individual with OSA by preventing them from getting appropriate amounts of sleep.
Treatment
Treatment options for obstructive sleep apnea generally fall into one of five different categories: lifestyle changes, breathing devices, oral appliances, surgical implant, and surgical procedures. Therapy to strengthen the muscles around the face and airway may also be an option.
Lifestyle Changes
- Regular physical activity
- Limit alcohol and caffeine intake
- Maintain a healthy weight
- Side sleeping (as opposed to sleeping on the back)
Breathing Devices
- Continuous positive airway pressure (CPAP) machine – A constant level of air pressure is delivered over the course of the night.
- Auto-adjusting positive airway pressure (APAP) machine – Air pressure is automatically adjusted based on the patient’s breathing patterns.
- Bilevel positive airway pressure (BiPAP) machine – Different air pressure levels are delivered for inhalation and exhalation.
Oral Appliances
Oral appliances for obstructive sleep apnea are most frequently used when patients do not want to use, or cannot tolerate, the breathing devices described above. There are a few main types of oral appliances:
- Mandibular repositioning mouthpieces – Holds the lower jaw in position in order to prevent it from blocking the upper airway
- Tongue-retaining devices – Holds the tongue in a forward position to prevent it from blocking the airway
- Neuromuscular electrical stimulation – Stimulates the tongue and upper airway muscles to prevent them from collapsing and blocking the airway during sleep4
Surgical Implant
Hypoglossal nerve stimulation utilizes a surgical implant to stimulate a nerve in order to prevent the tongue from blocking the airway. Inspire®, which was approved by the FDA in 2014, places implants in the neck and chest, and is controlled by a remote. Hypoglossal nerve stimulation may be an option for those with moderate-to-severe obstructive sleep apnea and who cannot tolerate, or do not benefit from, CPAP.
Surgical Procedures
Other surgical options exist to treat obstructive sleep apnea, which are usually considered when less invasive interventions have failed or are contraindicated.
- Uvulopalatopharyngoplasty (UPPP) – Surgical removal of tissue from the back of the mouth and top of the throat
- Maxillomandibular advancement – The jaw is moved forward to enlarge the space behind the tongue and soft palate, making obstruction less likely
- Nasal surgery – May remove polyps or straighten a deviated septum
- Tonsillectomy – To reduce snoring and improve airflow
Future Therapies
Research is ongoing to assess the safety and effectiveness of AD109, which would be the first oral medication to treat sleep apnea. AD109 combines the drugs aroxybutynin, currently used to treat overactive bladder, and atomoxetine, currently used to treat ADHD. Phase II studies “showed clinically meaningful improvement in OSA, suggesting that further development of the compound is warranted.”5
Another oral medication for sleep apnea, IHL‑42X, is also currently being assessed. IHL‑42X is a cannabinoid and is comprised of low-dose dronabinol (a synthetic form of THC) and acetazolamide (a carbonic anhydrase inhibitor).6 If approved by regulatory agencies, an oral medication option would be significantly meaningful for individuals who are unable to tolerate, or are not candidates for, other sleep apnea interventions.
Researchers are also examining the use of tirzepatide, the main ingredient in weight loss and type 2 diabetes medications Zepbound and Mounjaro, for obstructive sleep apnea. Roughly 70% of people who suffer from OSA also have obesity.
A recent study revealed that in people “with moderate-to-severe obstructive sleep apnea and obesity, tirzepatide reduced the AHI, body weight, hypoxic burden, hsCRP concentration, and systolic blood pressure and improved sleep-related patient-reported outcomes.”7 At least in part due to this study, Eli Lilly has applied for U.S. approval of Zepbound for sleep apnea (of note, the study referenced above was funded by Eli Lilly).
Summary
In spite of being the most common respiratory-related sleep disorder, obstructive sleep apnea remains significantly underdiagnosed. OSA can increase the risk of heart attack, stroke, depression, and impaired cognition, along with decreasing overall quality of life.
Numerous treatment options for OSA currently exist, including lifestyle modifications, breathing devices (e.g., CPAP), oral appliances, hypoglossal nerve stimulation, and various surgical interventions. Multiple new therapies are currently being evaluated as well, including oral medications and tirzepatide, the main ingredient in weight loss and type 2 diabetes medications Zepbound and Mounjaro.
Endnotes
- M. Melanie Lyons et al., “Global burden of sleep-disordered breathing and its implications”, Respirology 25(7): 690‑702, 21 May 2020, https://onlinelibrary.wiley.com/doi/10.1111/resp.13838.
- “Study Finds High Rate of Undiagnosed Sleep Apnea in Older Adults”, American Academy of Sleep Medicine (AASM) Foundation, 11 May 2018, https://foundation.aasm.org/aasm-foundation-study-published-jags.
- M. Li et al., “Association of sleep apnea and depressive symptoms among US adults: a cross-sectional study”, BMC Public Health 23(427), 6 March 2023, https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-15358-8.
- “FDA Authorizes Marketing of Novel Device to Reduce Snoring and Mild Obstructive Sleep Apnea in Patients 18 Years and Older,” U.S. Food and Drug Administration, 5 Feb. 2021, https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-novel-device-reduce-snoring-and-mild-obstructive-sleep-apnea-patients-18.
- Paula K. Schweitzer et al., “The Combination of Aroxybutynin and Atomoxetine in the Treatment of Obstructive Sleep Apnea (MARIPOSA): A Randomized Controlled Trial”, American Journal of Respiratory and Critical Care Medicine 208(12), 9 Oct. 2023, https://www.atsjournals.org/doi/full/10.1164/rccm.202306-1036OC.
- “Patient dosing commenced in RePOSA Phase 2/3 Clinical Trial Protocol to Assess IHL‑42X Drug in Patients with Obstructive Sleep Apnea”, Incannex Healthcare/GlobeNewswire, 30 May 2024, https://www.globenewswire.com/news-release/2024/05/30/2890648/0/en/Patient-dosing-commenced-in-RePOSA-Phase-2-3-Clinical-Trial-Protocol-to-Assess-IHL-42X-Drug-in-Patients-with-Obstructive-Sleep-Apnea.html.
- Atul Malhotra, M.D. et al., “Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity”, The New England Journal of Medicine 391(13), 21 June 2024, https://www.nejm.org/doi/10.1056/NEJMoa2404881.





